Abstract
Therapies for acute myeloid leukemia (AML) has barely changed over 30 years, while treatment for other blood cancers have made remarkable leaps forward. Recent advances in genomics have allowed molecular targeted therapies (i.e. FLT3-ITD, IDH, c-KIT inhibitors) extending survival, but most patients still succumb (Burd et al. Nat Med 2020). Therefore, developing more efficient, less toxic, and immune-based therapies for AML is an urgent unmet need. Previous studies showed that stromal cell-derived IL-33 stimulates myeloproliferative neoplasms (Mager et al. J Clin Invest 2015). Stimulation-2 (ST2), IL-33 receptor, contributes to leukemia stem cells (LSCs) survival in Cbfb-MYH11 mice (Wang et al . Sci Rep 2019). We showed that ST2 blockade enhanced graft versus leukemia activity against MLL-AF9 egfp AML after hematopoietic cell transplantation (Zhang et al . Sci Transl Med 2015). We and others, also, found that ST2 is expressed on normal murine and human hematopoietic stem cells (HSCs), respectively (Capitano et al. Blood Cells Mol Dis 2020; Alt et al. Biol Blood Marrow Transplant 2019). These data suggest a leukemia-promoting role of ST2/IL-33 signaling.
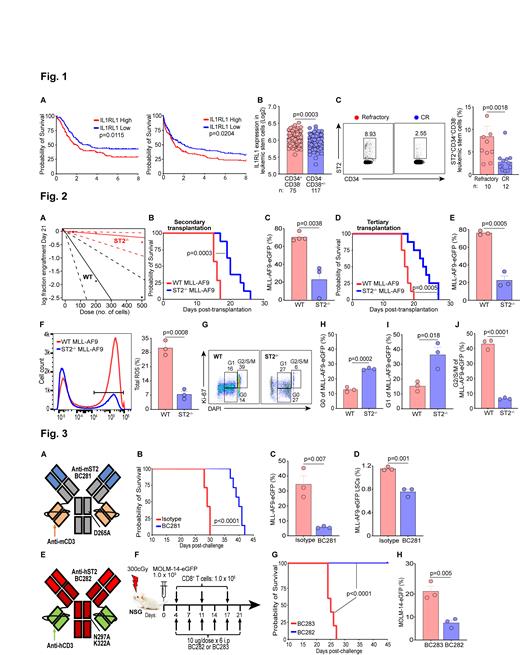
To determine clinical relevance of ST2 in AML, we generated Kaplan-Meier curves using TCGA (n=173) and TARGET AML (n=187) databases. Decreased survival was observed in patients with high IL1RL1 (ST2 gene) which was validated in an independent database (AMLCG 1999 trial, n=417) (Fig. 1A). Since ST2 is expressed on HSCs, we interrogated if ST2 is expressed on LSCs defined as CD34 +CD38 - in the Princess Margaret Leukemia biobank (n=192), and found ST2 is higher on LSCs vs CD34 -CD38 +/- cells (Fig. 1B). We then sought to analyze ST2 on bone marrow samples comparing complete responders vs refractory patients to note that ST2 expression was increased in refractory patients' LSCs (Fig. 1C). To scrutinize the role of ST2 in initiating leukemogenesis, we performed limiting dilution transplantation using 500, 200, and 50 Lin -Sca-1 +c-KIT +-sorted LSCs from WT vs ST2 -/- MLL-AF9 egfp transduced cells. Frequency of LSCs in ST2 -/- cells was decreased by ~15-fold as opposed to WT cells [1:2141 (1:546-1:8405) vs 1:145 (1:75-1:283), p=3.37e-05] (Fig. 2A). We also tested leukemia maintenance, secondary transplantations from the primary recipients resulted in leukemia growth delay in ST2 -/- vs WT cells which was confirmed in tertiary transplantations (Fig. 2B-E). Self-renewal ability of LSCs is correlated to reactive oxygen species (ROS) (Testa et al. Exp Hematol 2016), and we found that ROS levels in ST2 -/- leukemic cells are markedly diminished in contrast to WT leukemic cells (Fig. 2F). ST2 deficiency in leukemic cells arrests G2/S/M cell cycle progression in LSCs (Fig. 2G-J). These data indicated that ST2 is indispensable for initiating and maintaining LSCs in MLL-AF9 AML.
We next developed murine and human Fc-silenced-bispecific antibodies engaging mouse or human ST2 and CD3 (BsAb) built on the IgG[L]-scFv platform with proven ability to drive T cells into human tumors for effective tumor ablation (Santich et al. Sci Transl Med 2020; Park et al. J Immunother Cancer 2021) (Fig. 3A, 3E). Both BsAbs showed >90% purity by HPLC, stability under heat stress and low endotoxin. Animals did not exhibit any in vivo toxicity at BsAb doses of 0.4, 2, 5, 10 μg ip q 3 days x 6 doses (not shown). In the immunocompetent MLL-AF9 mice, murine anti-ST2 BsAb (BC281) treatment (10 μg i.p, 4 days post-AML challenge and given every three days for a total of 6 injections) resulted in extended survival compared to isotype control mice (Fig. 3B). Leukemic cells and LSCs were accordingly decreased in treated vs control group (Fig. 3C-D). We modeled humanized leukemic mice with MOLM-14 egfp cells and weekly injection of human CD8 + T cells in NOD.Cg-Prkdc scid Il2rg tm1Wjl/SzJ (NSG) mice (Fig. 3F). Animals treated with human anti-ST2 BsAb (BC282), using a similar regimen as for the immunocompetent model, led to better survival when compared to animals treated with mutated non-functional anti-ST2 BsAb (BC283) (Fig. 3G). Frequency of MOLM-14 egfp cells was lower in the BC282 vs BC283 group (Fig. 3H). These results suggested that anti-ST2 BsAbs can inhibit AML growth to improve survival.
We concluded that ST2 is a potential therapeutic target, and ST2-specific T cell engaging BsAbs represent promising immunotherapeutics for AML.
Cheung: Medical University of South Carolina: Patents & Royalties: inventor on the ST2 bispecific antibody patent application; Y-mabs Therapeutics and Abpro-Labs Inc: Patents & Royalties: inventor on multiple patents filed by MSK, including those licensed to Ymabs Therapeutics, Biotec Pharmacon, and Abpro-labs; Eureka Therapeutics: Membership on an entity's Board of Directors or advisory committees. Paczesny: Medical University of South Carolina: Patents & Royalties: inventor on the ST2 bispecific antibody patent application.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal